Ever wondered why some dying cells sound the alarm instead of going silent? Thats necroptosis a programmed, inflammatory form of cell death that cells unleash when theyre stuck in a nogo corner. In the next fifteen minutes youll find out exactly how the necroptosis pathway works, how it differs from the quieter apoptosis route, why it can be both a hero and a villain in disease, and what scientists are doing to tap into its power.
What Is Necroptosis?
Definition and basic trigger
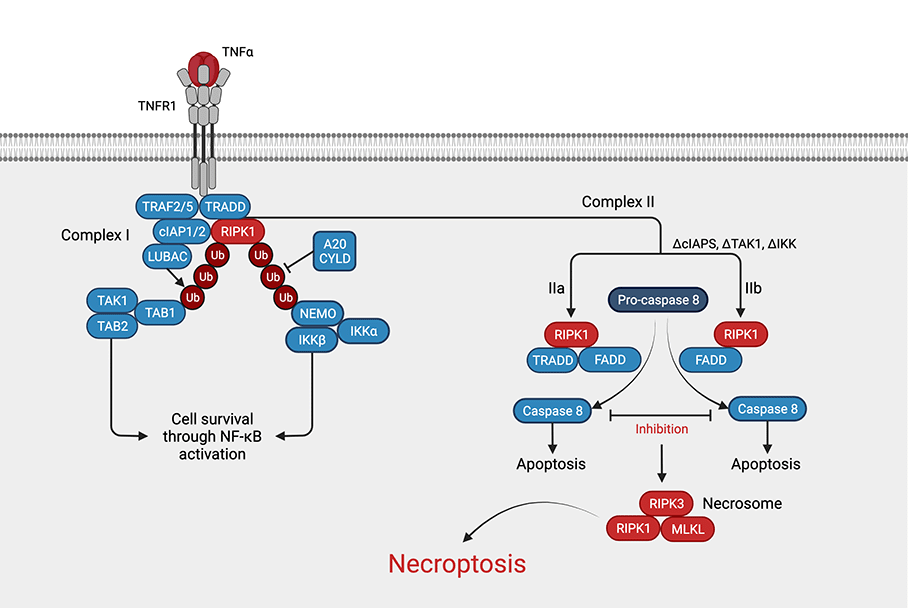
Necroptosis is a form of regulated cell death that combines the intentionality of apoptosis with the messiness of necrosis. Think of it as a controlled demolition that still leaves debris on the street. The pathway kicks in when deathreceptor signals (like TNF binding to TNFR1) meet a roadblock usually the inhibition or absence of caspase8. When that safety switch fails, the cell flips a different switch: the necroptosis mechanism.
Key players in the necroptosis pathway
The core engine includes three proteins that youll hear about a lot:
- RIPK1 the initial sensor that decides whether to go down the apoptosis or necroptosis road.
- RIPK3 the workhorse that partners with RIPK1 to form the socalled necrosome.
- MLKL the executioner. Once phosphorylated by RIPK3, it oligomerises and punches holes in the cells plasma membrane, spilling everything inside.
All of this happens while showed that the necroptosis pathway can be triggered by viral infections, DNA damage, or even certain chemotherapeutic drugs.
Detailed Necroptosis Mechanism
Stepbystep execution
1 Receptor activation TNF, FasL, or patternrecognition receptors (TLRs) recruit RIPK1 to the membrane.
2 Necrosome formation If caspase8 is blocked, RIPK1 binds RIPK3, forming a tight complex.
3 Phosphorylation of MLKL RIPK3 adds a phosphate group to MLKL, turning it into a lethal hammer.
4 Membrane rupture PhosphoMLKL translocates to the plasma membrane, creates pores, and the cell bursts, spilling inflammatory cytokines like IL1 and HMGB1.
How does necroptosis intersect with pyroptosis?
Both pathways end in a messy, inflammatory death, but the infantry differs. Pyroptosis relies on gasdermin pores after inflammasome activation, whereas necroptosis uses MLKL. Interestingly, found that in some immune cells, the two routes can crosstalk, amplifying the inflammatory response.
Necroptosis caspase relationship
The caspase8 switch is the pivotal decision point. When caspase8 is active, it cleaves RIPK1 and RIPK3, steering the cell toward apoptosis (the quiet, tidy death). In contrast, when caspase8 is inhibitedby viral proteins or pharmacological blockersthe necroptosis pathway takes the wheel.
Necroptosis vs Apoptosis
Morphological differences
| Feature | Necroptosis | Apoptosis |
|---|---|---|
| Cell size | Swelling (oncosis) | Shrinkage (pyknosis) |
| Membrane integrity | Loss, pore formation | Intact until later stages |
| Nuclear changes | Condensed chromatin, some fragmentation | Clear chromatin condensation, DNA laddering |
| Inflammation | High DAMPs released | Low silent clearance |
When does a cell choose necroptosis?
Imagine a city under siege. If the police (caspase8) are busy, the streetfighters (RIPK1/RIPK3/MLKL) take over, blowing up the doors to stop the invader even if it means collateral damage. In cellular terms, when upstream death receptors are engaged but caspase8 cant act, necroptosis becomes the default emergency exit.
RealWorld Examples
Necroptosis in neurodegeneration
In mouse models of ischemic stroke, researchers observed a dramatic surge in phosphoMLKL around the infarct core. Blocking RIPK3 with a smallmolecule inhibitor reduced the damaged area by roughly 30% (). This suggests that targeting the necroptosis pathway could be neuroprotective. For clinicians and researchers tracking therapeutic options, resources on atypical Rett syndrome highlight how modulating inflammatory cell death pathways can influence neurodevelopmental and neurodegenerative outcomes.
Cancer and necroptosis
Some aggressive tumors hijack necroptosis to create a hostile microenvironment that fosters metastasis. For example, a 2023 Cell Death & Disease paper reported that necroptotic extracellular vesicles carried proinflammatory signals that helped breastcancer cells seed distant organs. Yet, paradoxically, forcing necroptosis in certain leukemias can kill the cancer cells outright, showing the doubleedged nature of the pathway.
Infectious disease angle
Many viruses, like cytomegalovirus, produce proteins that block caspase8, intentionally nudging the host cell into necroptosis a move that can limit viral replication but also triggers inflammation. Understanding this tugofwar helps vaccine developers design smarter adjuvants.
Clinical Relevance Today
Benefits & risks of targeting the pathway
On the bright side, drugs that inhibit RIPK1 (e.g., necrostatin1 derivatives) are already in earlyphase trials for inflammatory skin disorders and amyotrophic lateral sclerosis. By damping the necroptosis pathway, they aim to reduce the fire that damages healthy tissue.
On the flip side, completely shutting down necroptosis could weaken our innate defense against certain pathogens. Its like turning off the fire alarm because the alarm itself sometimes causes water damage. A balanced approachpartial inhibition or contextspecific targetingappears to be the safest bet.
Emerging therapeutics
| Agent | Target | Clinical Status | Primary Indication |
|---|---|---|---|
| GSK872 | RIPK3 inhibitor | Preclinical | Ischemic injury |
| Necrosulfonamide | MLKL blocker | Preclinical | Autoimmune disease |
| Necrostatin1 (optimized) | RIPK1 inhibitor | Phase I/II | ALS, psoriasis |
How to pronounce necroptosis
Its neckROtosis. A quick tip: say neck + row + tosis and youll sound like a seasoned scientist.
Keeping UptoDate
Key journals and alerts
For the freshest breakthroughs, follow Nature Cell Biology, Cell Death & Disease, and the annual International Congress on Cell Death. Setting up a PubMed RSS feed for necroptosis pathway will drop new papers straight into your inbox. Trust me, staying informed feels like having a backstage pass to the molecular theater.
Community resources
Several labs host opensource necroptosis toolkits on GitHub, complete with CRISPR guides for RIPK3 and MLKL knockout. Engaging with these communities on platforms like ResearchGate can help you troubleshoot experiments and even spark collaborations.
Conclusion
The necroptosis pathway is a tightly regulated, inflammatory form of cell death that sits at the crossroads of immunity and disease. By understanding its triggers, core proteins (RIPK3, MLKL), and how it diverges from the serene apoptosis route, we gain a powerful lens to view conditions ranging from stroke to cancer. Whether youre a budding researcher, a clinician, or just a curious mind, keeping an eye on emerging inhibitors and clinical trials will be key as this field moves from bench to bedside. Got thoughts or experiences with necroptosis in your work? Share them below lets keep the conversation alive!
FAQs
What triggers the necroptosis pathway?
Necroptosis is activated when death‑receptor signals (e.g., TNF‑α) encounter a blocked or missing caspase‑8, allowing RIPK1 and RIPK3 to form the necrosome.
How does necroptosis differ from apoptosis?
Necroptosis leads to cell swelling, membrane rupture and release of inflammatory DAMPs, whereas apoptosis involves cell shrinkage, intact membranes and silent clearance.
Which proteins are the core executors of necroptosis?
The key players are RIPK1 (sensor), RIPK3 (necrosome scaffold) and MLKL (executioner that perforates the plasma membrane after phosphorylation).
Can necroptosis be a therapeutic target?
Yes. Inhibitors of RIPK1, RIPK3 or MLKL are in development for conditions such as stroke, ALS, inflammatory skin disease and certain cancers.
Is necroptosis always harmful?
No. While it can exacerbate inflammation in neurodegeneration or cancer, it also serves as an antiviral defense by killing infected cells that evade apoptosis.