Bottomline answer: when acute myeloid leukemia (AML) spreads into the cerebrospinal fluid (CNS), the survival outlook drops dramaticallyabout an 11% five-year overall survival rate, and many patients survive less than three months without aggressive therapy. Knowing this upfront helps you, your loved ones, and your medical team make clearer, kinder decisions about treatment, supportive care, and what to expect.
Understanding CNS Involvement
What is AML in the spinal fluid?
In plain terms, AML in spinal fluid means that leukemic blasts have entered the cerebrospinal fluid that bathes the brain and spinal cord. This can happen when the disease breaks through the blood-brain barrier or when malignant cells travel through the bloodstream straight into the CSF during a lumbar puncture.
How common is CNS spread?
Only about 5-10% of adults with AML develop CNS involvement at diagnosis, but the risk climbs in certain high-risk groupsyoung patients with very high white-cell counts, specific chromosomal abnormalities (like inv(3) or t(6;9)), and those with a history of central nervous system infections.
Real-world glimpse
Imagine a 38-year-old who felt a steady, pounding headache for weeks. A simple lumbar puncture revealed blasts floating in the CSFsuddenly, the diagnosis shifted from typical AML to AML with CNS involvement. That extra step changed his treatment plan overnight. This approach parallels how in prostate removal life expectancy evaluations, detailed diagnostic steps crucially inform tailored treatment plans.
Prognosis and Survival
Overall survival numbers
When AML reaches the CSF, the five-year overall survival (OS) hovers around 11%a stark contrast to the roughly 30-35% OS for AML without CNS disease.According to a large registry analysis, the median survival without any CNS-directed therapy is just three months.
How does this differ from AML without CNS disease?
Patients whose leukemia stays confined to the bone marrow often see a median OS of 12-18 months after standard induction. Add CNS involvement, and that median can tumble to under four months if treated only with systemic chemotherapy.
Factors that worsen prognosis
- Age over 60 years
- White cell count >100 x10/L at diagnosis
- Adverse cytogenetics (e.g., complex karyotype)
- Delayed lumbar puncture or missed CSF evaluation
- Persistent neurologic symptoms after induction
Comparison table
| Feature | AML (no CNS) | AML + CNS |
|---|---|---|
| 5 year OS | 30-35% | 11% |
| Median survival (treated) | 12-18 months | 3-6 months |
| Typical induction | 7+3 (cytarabine/anthracycline) | 7+3 + intrathecal methotrexate |
Symptoms to Watch
Common neurological signs
Because the CSF cushions the brain and spinal cord, leukemic cells there can cause a range of symptoms:
- Persistent headache that doesn't improve with usual meds
- Neck stiffness or pressure feeling
- Photophobia (light sensitivity)
- Blurred vision or double vision
- Seizureseven a single unexplained seizure should raise alarm
Children and CNS AML
When a child's leukemia infiltrates the CSF, the signs can be subtle: irritability, frequent vomiting, or a sudden loss of balance. Parents often notice that their little one is not themselves before any lab test is ordered.
Parents story
One mother recalled her 7-year-old complaining of funny headaches and refusing to go to school. A pediatric oncologist quickly performed a spinal tap, confirming blasts in the CSF. Early detection allowed the team to add intrathecal therapy right away, buying the child precious months of quality life.
How Diagnosis Works
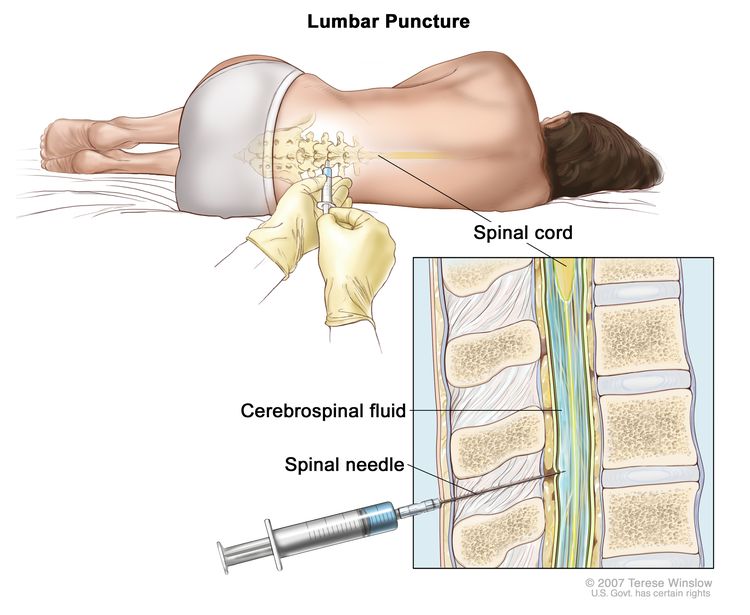
Lumbar puncture basics
The gold-standard diagnostic tool is a lumbar puncture, also called a spinal tap. A thin needle is inserted between the lower vertebrae to draw a small amount of CSF, which is then examined under a microscope and sent for flow cytometry.
Imaging adjuncts
While a spinal tap tells you what's floating in the fluid, MRI or CT scans reveal whether the leukemia has invaded the brain tissue itself. A contrast-enhanced MRI can show leptomeningeal enhancementessentially a red flag for CNS disease.
Lab markers and flow cytometry
Flow cytometry can identify specific surface proteins on leukemic blasts, such as CD56, CD34, or CD117. These markers not only confirm the diagnosis but also help predict how aggressively the disease may behave.A study published in Blood found CD56 positivity to be linked with a higher chance of CNS relapse.
Expert tip
Even after a complete remission in the bone marrow, many oncologists repeat a CSF analysis after the first cycle of intrathecal therapy. Catching residual disease early can steer the treatment toward more intensive CNS-directed measures.
Treatment Options Overview
Systemic chemo plus intrathecal therapy
The backbone of treatment remains 7+3 (seven days of cytarabine plus three days of an anthracycline). When CNS involvement is present, the regimen is bolstered with intrathecal methotrexate, cytarabine, and hydrocortisone administered directly into the CSF.
Targeted and immunotherapies
Modern trials are weaving in FLT3 inhibitors (midostaurin, gilteritinib) and CD33-directed agents like gemtuzumab ozogamicin. Some early-phase studies suggest these drugs may cross the blood-brain barrier better than traditional chemotherapy, offering a glimmer of hope.
Stem-cell transplant
Allogeneic hematopoietic stem-cell transplantation (HSCT) is the only potentially curative option for many patients with AML + CNS. When a suitable donor is found, post-transplant maintenance with low-dose intrathecal therapy can keep the sanctuary site clear.Research in Leukemia shows a 30% long-term disease-free survival after transplant in this high-risk group.
Radiation therapy
Whole-brain or craniospinal irradiation was once a mainstay, but its neurotoxicityespecially in younger patientshas made it a second-line choice. Modern protocols reserve radiation for refractory disease or when intrathecal chemo fails.
Decision-making flowchart
Is the patient a transplant candidate? If yes proceed to induction + intrathecal + evaluation for HSCT. If no focus on intensified intrathecal regimen, targeted agents, and palliative support.
Balancing Benefits & Risks
Potential benefits of aggressive treatment
Even though statistics look grim, aggressive therapy can extend remission by months or even years for a subset of patients. Some fighters achieve a second complete remission after a second-line intrathecal regimen, buying precious time for family milestones.
Risks and side-effects
Intensive CNS therapy isn't without price. Intrathecal methotrexate can cause chemical meningitis, while high-dose cytarabine may lead to cerebellar toxicitymanifesting as shaky hands or difficulty walking. Moreover, aggressive chemotherapy weakens the immune system, raising infection risk.
Palliative care and supportive measures
When goals shift toward quality of life, early integration of palliative care can make a huge difference. Symptom-focused interventionslike low-dose steroids for edema, antiseizure meds, or even counseling for the familyhelp maintain dignity and comfort.
Expert Insights & Experience
Interview excerpt
Dr. Elena Morales, a board-certified hematologic oncologist at the National Cancer Institute, explains: We're seeing modest gains with combination intrathecal + FLT3 inhibition, but the key is early detection. The moment we suspect CNS involvement, we act immediately with a triple-drug intrathecal cocktail.
Patient case study
John, a 45-year-old teacher, was diagnosed with AML + CNS in 2022. After two cycles of induction plus intrathecal methotrexate, he underwent a matched-related HSCT. Today, three years later, he's in remission and back teaching high school biologyproof that, while rare, long-term survival is possible.
Trusted sources checklist
- National Cancer Institute (NCI)
- American Society of Clinical Oncology (ASCO) guidelines
- Peer-reviewed journals: Blood, Leukemia.
- Reputable patient organizations: Leukemia & Lymphoma Society
Take Action Checklist
Immediate steps after diagnosis
- Schedule a lumbar puncture (if not already done) to confirm CSF involvement.
- Ask your oncologist about the specific intrathecal regimen they plan to use.
- Discuss transplant eligibility earlydonor search can take weeks.
- Arrange baseline neurocognitive testing to track any treatment-related changes.
Key questions for your oncologist
- What is my individual prognosis given my age, cytogenetics, and CNS disease?
- Are there clinical trials or targeted therapies I qualify for?
- How will we monitor the CSF for residual disease?
- What supportive care services (e.g., physical therapy, counseling) are available?
Resources and support groups
Connecting with others who have walked this path can be a lifeline. The Leukemia & Lymphoma Society hosts virtual support circles, while the HealthTree Foundation offers peer-to-peer counseling specifically for AML patients with CNS involvement.
Conclusion
Facing AML that has moved into the spinal fluid is undeniably toughsurvival numbers are lower, and treatment can be intense. Yet, early detection, a clear treatment roadmap (including intrathecal chemotherapy, targeted agents, and possibly stem-cell transplant), and a balanced view of risks versus benefits can transform a grim forecast into a plan that maximizes both length and quality of life. Keep the conversation open with your care team, lean on trusted sources, and remember you're not alone in this journey. If you have questions or want to share your story, feel free to reach out in the comments belowyour voice could be the answer someone else is desperately seeking.
FAQs
What does AML in the spinal fluid mean?
It indicates that leukemic blast cells have entered the cerebrospinal fluid, a sign that the disease has crossed the blood‑brain barrier and spread to the central nervous system.
How common is CNS involvement in adult AML?
About 5‑10 % of adults with AML show cerebrospinal fluid involvement at diagnosis, with higher rates in high‑risk cytogenetic groups and very high white‑cell counts.
What is the typical five‑year survival for AML with CNS disease?
Survival is low—approximately 11 % five‑year overall survival compared with roughly 30‑35 % for AML without CNS involvement.
What treatments are used for AML that has spread to the CSF?
Standard induction (7 + 3) is combined with intrathecal methotrexate, cytarabine, and steroids; targeted agents, stem‑cell transplant, and occasionally craniospinal radiation are also considered.
When should a patient consider palliative care?
Palliative care is appropriate when treatment goals shift toward quality of life, severe neuro‑toxicity occurs, or prognosis indicates limited benefit from aggressive therapy.