Quick Answer Overview
TNF necroptosis is a form of programmed cell death that kicks in when the inflammatory signal TNF (tumor necrosis factoralpha) binds its receptor, flips a molecular switch, and forces the cell to burst open in a messy, inflammatory way. It matters because this explosive death can drive brainshaking diseases like Alzheimers and stroke, but it also offers a target for drugs that could calm down harmful inflammation.
Core Necroptosis Science
What Triggers Necroptosis?
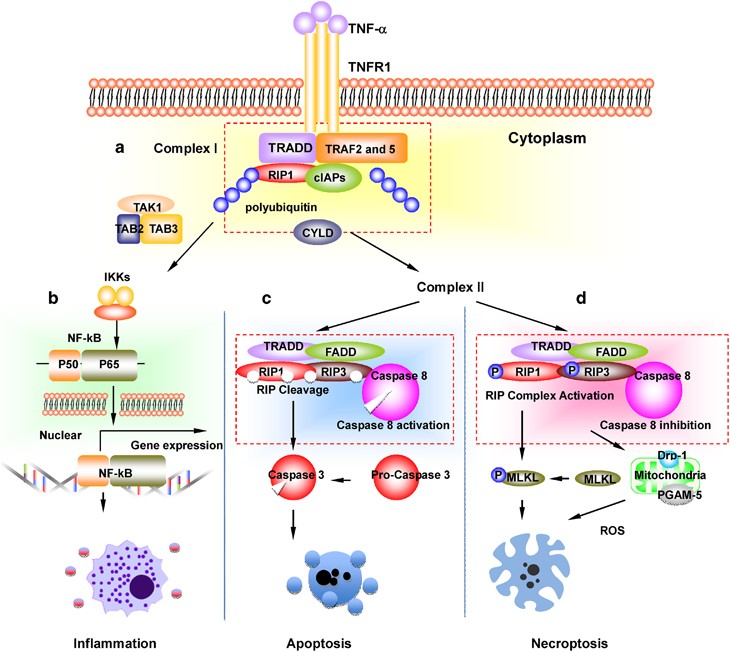
Think of TNF as a fire alarm. When it lands on the TNFR1 receptor, it summons a crew of adaptor proteins (TRADD, TRAF2/5) that decide whether the cell lives, repairs itself, or blows up. If caspase8 the usual executioner of apoptosis is blocked or inhibited, the alarm shifts to the necrosome team: RIPK1, RIPK3, and the executioner MLKL. Thats the moment necroptosis gets a green light.
StepbyStep Mechanism
1. TNF binds TNFR1 a signaling complex forms.
2. Ubiquitination status of RIPK1 determines fate: ubiquitinated RIPK1 keeps the cell alive, deubiquitinated RIPK1 joins the necrosome.
3. RIPK1 partners with RIPK3, and they phosphorylate each other.
4. MLKL gets phosphorylated (Ser358/Ser357) and oligomerizes.
5. MLKL oligomers crawl to the plasma membrane, pierce it, and the cell swells and bursts, spilling its contents and sounding an inflammatory alarm.
Key Players MLKL and RIPK3
MLKL (mixed lineage kinaselike) is the executioner with a fourhelix bundle that, once phosphorylated, flips open like a springloaded door. RIPK3 is the bridge that shuttles the phosphate group from RIPK1 to MLKL. Without either protein, the necroptotic cascade stalls, and the cell may default back to apoptosis or survive.
Morphology of Necroptosis
Under the microscope, necroptotic cells look like swollen balloons organelles puff up, the plasma membrane becomes porous, and the cell eventually ruptures. Unlike the tidy DNA fragmentation of apoptosis, necroptosis leaves a chaotic mess that draws immune cells to the scene.
Comparison of CellDeath Types
| Feature | Necroptosis | Apoptosis | Pyroptosis |
|---|---|---|---|
| Trigger | TNF, RIPK1/3, caspase8 inhibition | Intrinsic/Extrinsic caspase activation | Inflammasome, caspase1/11 |
| Key Executer | MLKL | Caspase3/7 | GasderminD |
| Morphology | Cell swelling, membrane rupture | Cell shrinkage, apoptotic bodies | Cell swelling, pore formation |
| Inflammation | High (DAMP release) | Low (silent clearance) | High (IL1, IL18 release) |
| DNA Fragmentation | Minimal | Extensive | Limited |
Real World Examples
Alzheimers Disease
In mouse models of Alzheimers, researchers have seen neurons light up for phosphoMLKL after chronic TNF exposure a classic necroptosis example. The resulting balloonlike neurons contribute to synaptic loss and memory decline. A study in describes how inhibiting RIPK1 can rescue those neurons, hinting at a therapeutic angle.
Vascular Endothelium
When microglia release TNF during neuroinflammation, brain bloodbrainbarrier (BBB) endothelial cells can undergo necroptosis, compromising barrier integrity. The resulting leak lets peripheral immune cells flood in, amplifying damage. This mechanism was highlighted in a recent paper on strokerelated BBB breakdown.
Cancer Cell Death
Some tumors dodge apoptosis but remain vulnerable to necroptosis. In breast cancer cells, TNF can push the necroptosis pathway, especially when mitochondrial superoxide spikes. This doublehit strategyTNF plus ROScreates a necroptosis example that researchers are exploiting to design smarter chemo combos ().
Neuropathic Pain
Chronic pain often stems from glial activation and TNF release. A recent MDPI review showed that peripheral nerve injury triggers MLMLdependent necroptosis in dorsal root ganglion neurons, feeding the pain loop. Blocking RIPK1 or MLKL in animal models dampened pain behaviors, suggesting a new analgesic pathway.
Compare Cell Death
Necroptosis vs Apoptosis
Apoptosis is the quiet suicide: caspases slice DNA, the cell shrinks, and phagocytes clean up without a fuss. Necroptosis, by contrast, is noisy it bursts the membrane, releases damageassociated molecular patterns (DAMPs), and calls in immune cells. Both can be initiated by TNF, but the presence or absence of active caspase8 decides which road you travel.
Necroptosis vs Pyroptosis
Both are inflammatory, but pyroptosis hinges on inflammasomes and gasderminD pores, usually in response to bacterial toxins. Necroptosis uses the RIPKMLKL axis and can be triggered by cytokines like TNF. While pyroptosis often releases IL1 and IL18, necroptosis dumps a broader mix of DAMPs, making it a more general danger signal.
Therapeutic Possibilities
Inhibitors in the Pipeline
Scientists have crafted necrostats such as Nec1s, which latch onto RIPK1s kinase pocket and stop the necroptotic switch. Newer MLKL inhibitors are still in preclinical stages, but early data show they can protect neurons in mouse models of traumatic brain injury. These compounds illustrate how targeting the TNF necroptosis pathway could dampen harmful inflammation without shutting down normal immune defenses.
Balancing Benefits and Risks
Turning off necroptosis isnt a free lunch. The pathway helps clear infected or damaged cells, so a blanket inhibition might leave the body vulnerable to lingering infections. The key is a balanced approachtemporarily blocking RIPK1 during a flareup of neuroinflammation, then letting the system reset.
Clinical Trials Snapshot
Several PhaseI/II trials are now testing RIPK1 inhibitors (e.g., DNL747) in Alzheimers and amyotrophic lateral sclerosis. Early reports suggest good safety profiles, but efficacy data are still pending. You can peek at the trial details on the , which lists inclusion criteria, dosing, and outcome measures.
Key Takeaways
TNF necroptosis sits at a crossroads of inflammation and cell death. When TNF blunts caspase8, the RIPK1RIPK3MLKL cascade fires, causing cells to swell and burst a process that fuels diseases like Alzheimers, stroke, and certain cancers, yet also offers a promising drug target. Understanding both the upside (clearing dangerous cells) and the downside (excessive inflammation) helps us think critically about future therapies. If youre curious about the latest necroptosis inhibitors or have questions about how this pathway might relate to a condition you care about, feel free to drop a comment below or subscribe for updates. Lets keep the conversation going and explore how science can turn a messy cellular explosion into a chance for healing.
For clinicians and patients navigating rare disease treatments and support options, resources on assistance programs can be useful; for example, information about Exondys 51 assistance can help families understand access pathways for therapies in neuromuscular disorders.
FAQs
What is TNFα necroptosis?
TNFα necroptosis is a form of programmed necrotic cell death initiated when TNF‑α binds its receptor and the caspase‑8 pathway is blocked, activating the RIPK1‑RIPK3‑MLKL cascade.
How does the necroptosis pathway differ from apoptosis?
Necroptosis uses the RIPK‑MLKL axis and results in cell swelling and membrane rupture, releasing inflammatory signals, whereas apoptosis relies on caspases, leads to cell shrinkage and is generally non‑inflammatory.
Which diseases are most associated with TNFα‑driven necroptosis?
Research links TNFα necroptosis to neurodegenerative disorders such as Alzheimer’s disease, acute brain injuries like stroke, certain cancers, and chronic neuropathic pain.
Are there drugs that can inhibit TNFα necroptosis?
Yes, small‑molecule necrostatins (e.g., Nec‑1s) target RIPK1, and experimental MLKL inhibitors are being developed; several RIPK1 inhibitors are in early clinical trials for neuroinflammatory diseases.
Can blocking necroptosis have side effects?
Because necroptosis helps remove infected or damaged cells, long‑term inhibition may increase susceptibility to infections or impair tissue remodeling, so therapies aim for selective or time‑limited dosing.