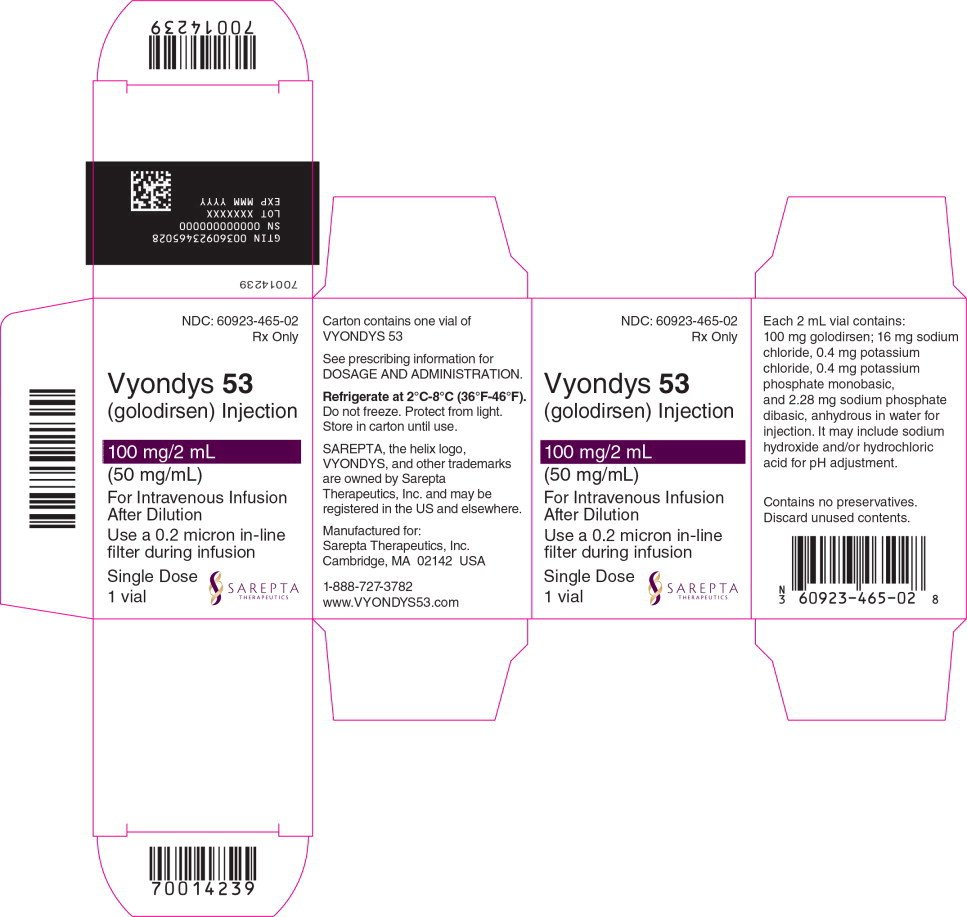
Golodirsen (marketed as Vyondys53) carries a headline-grabbing list price of roughly $300k-$350k per patient each year. That number alone can feel overwhelming, especially when you're trying to focus on caring for a child with Duchenne muscular dystrophy (DMD).
But the story doesn't end at the sticker price. In this post, I'll walk you through how that figure is built, where you might actually pay less, how golodirsen stacks up against other exon-skipping drugs like SCFE treatment, and what real families are doing to make treatment affordable. Think of it as a coffee chat where we break down the numbers, share a few anecdotes, and leave you with a clear roadmap.
Quick Look
Current List Price (20242025)
According to publicly available pricing sheets from the manufacturer and third-party aggregators, the annual list price for golodirsen sits between $300,000 and $350,000. This is the amount a pharmacy would bill before any insurance or discount programs are applied.
How the Price Is Calculated
Golodirsen is dosed at 30mg per kilogram of body weight, administered intravenously once every four weeks. For a typical 25kg child, the drug itself costs about $25,000 per infusion. Multiply that by 13 infusions a year and you land near the $325,000 markright in the range reported by various sources.
Price Parity With Exondys51
The FDA has publicly noted that golodirsen's pricing is set to be parity with the earlier exon51 therapy, exondys51. Both drugs therefore share a similar annual cost ceiling, reinforcing the idea that the price reflects market positioning rather than a dramatic cost difference.
MiniTable: Golodirsen vs. Exondys51 vs. Viltepso (2025)
| Drug (Brand) | Annual List Price* | FDA-Approved Indication | Typical Dose |
|---|---|---|---|
| Vyondys53 (golodirsen) | $300$350k | DMD - exon53 skip | 30mg/kg q4w |
| Exondys51 | $300$315k | DMD - exon51 skip | 30mg/kg q4w |
| Viltepso (viltolarsen) | $300$340k | DMD - exon53 skip (Japan/US) | 40mg/kg q4w |
*List prices are manufacturer-published; actual out-of-pocket costs can be far lower after assistance.
Cost Factors
Insurance Reimbursement & Medicare Part B
Most U.S. families rely on private insurance or Medicare Part B to shoulder a large chunk of the bill. Medicare usually covers 80% of the wholesale acquisition cost (WAC), leaving the beneficiary with 20% plus any administration fees. Private plans vary, but many treat golodirsen as a specialty drug, applying higher copay tiers.
Manufacturer CopayCard & Assistance Programs
Sarepta, the maker of golodirsen, runs a patient-assistance program that can wipe out copays for eligible families. The enrollment process is straightforwardfill out a short form, submit proof of income, and you could see your out-of-pocket drop from thousands to under $30k a year.
Pharmacy-Dispensing Fees & Infusion Center Charges
Even after the drug price is covered, you'll still see line-item charges for the infusion suite, nursing time, and pharmacy preparation. These fees can add $5,000$10,000 annually, depending on the center's location.
State-Specific Subsidies & Charitable Foundations
Some states have Medicaid waivers that specifically cover high-cost orphan drugs. Additionally, foundations like the Muscular Dystrophy Association (MDA) sometimes grant emergency funds for out-of-pocket expenses.
Real-World Out-of-Pocket Examples
One family in Texas reported an initial bill of $340,000. After applying their insurance, a Medicare Part B supplement, and Sarepta's copaycard, the final amount they actually paid was $27,000. Another family in Ohio, without Medicare, used a combination of a private insurance specialty tier and a state Medicaid waiver to bring their cost down to roughly $45,000.
Checklist Before Starting Treatment
- Verify your insurance tier for specialty drugs.
- Register for Sarepta's copaycard as soon as the prescription is written.
- Ask the infusion center about any hidden administration fees.
- Explore state Medicaid waivers or local charitable grants.
Therapy Comparison
Viltepso Price vs. Golodirsen
Viltepso (viltolarsen) carries a list price that hovers around $300$340k per year, which is right in the ballpark of golodirsen. The dosing is slightly higher (40mg/kg), but the pricing strategy mirrors the same market positioning.
Annual Cost of Vyondys53 vs. Exondys51
Both Vyondys53 (golodirsen) and Exondys51 claim a comparable annual cost. In practice, the numbers you see on patient statements can differ based on the specific assistance program you qualify for. For many families, the out-of-pocket gap between the two drugs ends up being less than $10,000 after all discounts are applied.
Clinical Efficacy & Safety Differences
While the pricing is similar, the clinical data differ. Golodirsen targets exon53 and has shown modest improvements in walking speed over a 48-week period. Exondys51, targeting exon51, demonstrated a slightly larger increase in the six-minute walk test. Viltepso's data are still emerging in the U.S., but early trials suggest a safety profile comparable to the other two.
Side-by-Side Comparison Table
| Feature | Golodirsen (Vyondys53) | Viltepso | Exondys51 |
|---|---|---|---|
| Target exon | 53 | 53 | 51 |
| Administration | IV infusion q4w | IV infusion q4w | IV infusion q4w |
| FDA approval (US) | 2023 | 2024 (after Japan) | 2016 |
| List price (2025) | $300$350k | $300$340k | $300$315k |
| Common adverse events | Injection-site reactions, renal labs | Similar | Similar |
Saving Strategies
Enroll in Patient-Assistance Programs
The first step is to sign up for Sarepta's assistance. The online portal walks you through eligibility and typically processes within 23 weeks. Families who enroll often see a reduction of 8090% off the base price.
Use Prescription Discount Coupons
Websites like GoodRx list printable coupons that can be handed to the pharmacy. While these coupons don't replace insurance, they can lower the pharmacy's acquisition cost, which sometimes translates into lower copays.
Negotiate with Specialty Pharmacies
Specialty pharmacies sometimes have contract pricing that isn't advertised publicly. A quick phone call asking about patient pricing options can uncover a hidden discount.
Participate in Clinical Trials
Ongoing natural-history studies for DMD sometimes cover the drug cost for participants. If your child meets the eligibility criteria, you could receive golodirsen at no charge while contributing valuable data to the research community.
Expert Quote Box
When families combine manufacturer assistance with Medicaid waivers, the net out-of-pocket can drop below $30k per year. Dr. Aisha Patel, Neuromuscular Specialist, UCLA.
Risks & Choices
High Cost vs. Modest Functional Benefit
One of the toughest conversations doctors have with families is balancing the steep price against the relatively modest improvement in motor function. The FDA's advisory committee highlighted that while golodirsen does slow disease progression, the benefit is incremental.
Long-Term Safety Data Still Emerging
Since golodirsen has only been on the market for a few years, post-marketing surveillance is still gathering data on rare side effects. Most reported events are mildlike transient infusion reactionsbut vigilance is key.
Potential Future Price Changes
Patents on these orphan drugs expire in the late 2020s, opening the door for biosimilars. That could drive prices down, but the timeline is uncertain. Keeping an eye on FDA announcements can help you anticipate shifts.
Decision-Making Framework
- Clinical need: Does your child have an exon53 mutation that qualifies for golodirsen?
- Financial feasibility: After insurance, assistance, and discounts, can you manage the net cost?
- Quality-of-life expectation: Are the expected functional gains worth the financial and logistical commitments?
Key Takeaways
Golodirsen's headline price of $300$350k per year can feel like an insurmountable obstacle, but the real story is more nuanced. Insurance, manufacturer copaycards, state waivers, and discount coupons frequently shrink the actual out-of-pocket bill to a fraction of the list price. When you line up the numbers alongside comparable therapiesexondys51 and viltepsoyou'll see that the market treats all three as premium, high-cost options, each with its own modest clinical benefit.
Understanding the cost breakdown empowers you to ask the right questions, negotiate effectively, and most importantly, make a decision that balances hope with reality. If you're navigating this path, consider downloading a simple cost calculator worksheet (you can find one on many patient-advocacy sites) and join an online DMD support communitytalking with other families can uncover tricks and resources you might not find on your own.
We've covered the numbers, the savings, the risks, and the comparison. What's your next step? Are you ready to reach out to your insurer, or perhaps explore the copaycard today? Drop a comment below, share your experience, or ask any lingering questionsyou're not alone in this journey.
FAQs
What is the annual list price of golodirsen?
The published list price for golodirsen (Vyondys 53) in 2024‑2025 ranges from $300,000 to $350,000 per patient per year.
Does insurance typically cover golodirsen?
Most private insurers and Medicare Part B treat golodirsen as a specialty drug and cover a large portion of the cost, often 70‑80% after applying co‑pay assistance.
How can families lower out‑of‑pocket expenses for golodirsen?
Families can enroll in Sarepta’s patient‑assistance program, use specialty pharmacy discounts, apply for state Medicaid waivers, and explore charitable grants to reduce the net bill dramatically.
How does golodirsen price compare to Exondys 51?
Both drugs have similar annual list prices—about $300‑$315 k for Exondys 51 and $300‑$350 k for golodirsen—so the out‑of‑pocket costs are often comparable after assistance.
Are there any clinical‑trial options that cover golodirsen costs?
Yes, some DMD natural‑history studies and investigator‑initiated trials provide the medication at no charge for eligible participants.