Did you know that the tiny warning on your cough syrup could be the difference between a quick recovery and an unexpected emergency? In just a few seconds you can spot the redboxed text, a nicotine graphic on a cigarette pack, or a plainEnglish caution on a medical device. Those warnings arent there for show theyre the FDAs way of putting your safety first.
In this post Ill walk you through everything you need to know about the FDA warning label from why it exists, to how companies get them, and what you as a consumer (or business owner) should be looking for. No jargon, no fluff, just the realworld info that helps you make smarter choices.
Quick Overview
What is an FDA warning label?
An FDA warning label is a mandated statement that appears on a products packaging or accompanying literature to alert users about serious risks, contraindications, or required precautions. It can be a boxed warning (the black box on prescription drugs), a nicotinespecific warning on tobacco products, or a simple caution on a cosmetic.
Why should you care?
Because those warnings are legally enforceable. Ignoring them can mean higher chances of adverse reactions, legal liability for manufacturers, and even product recalls. For you, its peace of mind you know exactly what to watch out for before you swallow, inhale, or apply something.
How does it differ from a caution statement?
A caution is a softer advisory, often used for minor side effects. A warning signals a serious or lifethreatening risk. The FDA reserves the word warning for highimpact safety information, while caution covers lowerseverity issues.
Warning Letter Ecosystem
What is an FDA warning letter?
When the FDA finds a product or facility thats out of compliance, it issues a formal . Its a public notice that the company must correct the problem within a set timeframe, or face further enforcement actions.
Types of FDA warning letters
The agency categorizes its letters to help both regulators and the public understand the severity:
- Form 483 observations: Preliminary findings noted during an FDA inspection.
- Compliancerelated letters: Issues like labeling errors, adulterated products, or unapproved claims.
- Safetyrelated letters: Direct threats to consumer health, such as missing warnings.
- Marketingrelated letters: Misleading advertisements or promotion of offlabel uses.
Where to find the FDA warning letter database?
The FDA maintains a searchable warningletter database where you can pull up recent letters by product type, company name, or date. Simply visit the FDAs and use the filter tools.
How to read a warning letter effectively?
Focus on three sections:
- Observations: What the FDA found wrong.
- Regulatory citations: Which laws or guidances were violated.
- Corrective actions: What the company must do, and by when.
Spotting the regulatory citations helps you trace back to the exact FDA warning label requirements that were breached.
Labeling Requirements
The FDAs labeling mandates vary by product category, but they all share the same core principle: clear, prominent, and truthful risk communication.
| Product Category | Key Warning Requirement | Typical Placement |
|---|---|---|
| Prescription Drugs | Boxed warning for serious risks; standard warning text for contraindications. | Prominent box on the prescribing information; highlighted on the outer label. |
| Tobacco & Nicotine | Graphic health warnings covering at least 30% of the principal display area. | Front and back of cigarette packs; on vaping device packaging. |
| Medical Devices | Warnings for devicespecific hazards; Warnings and Precautions section in IFU. | Package insert; device label. |
| Food & Dietary Supplements | Allergen statements; Do not use if warnings for highrisk populations. | Front label or supplemental information sheet. |
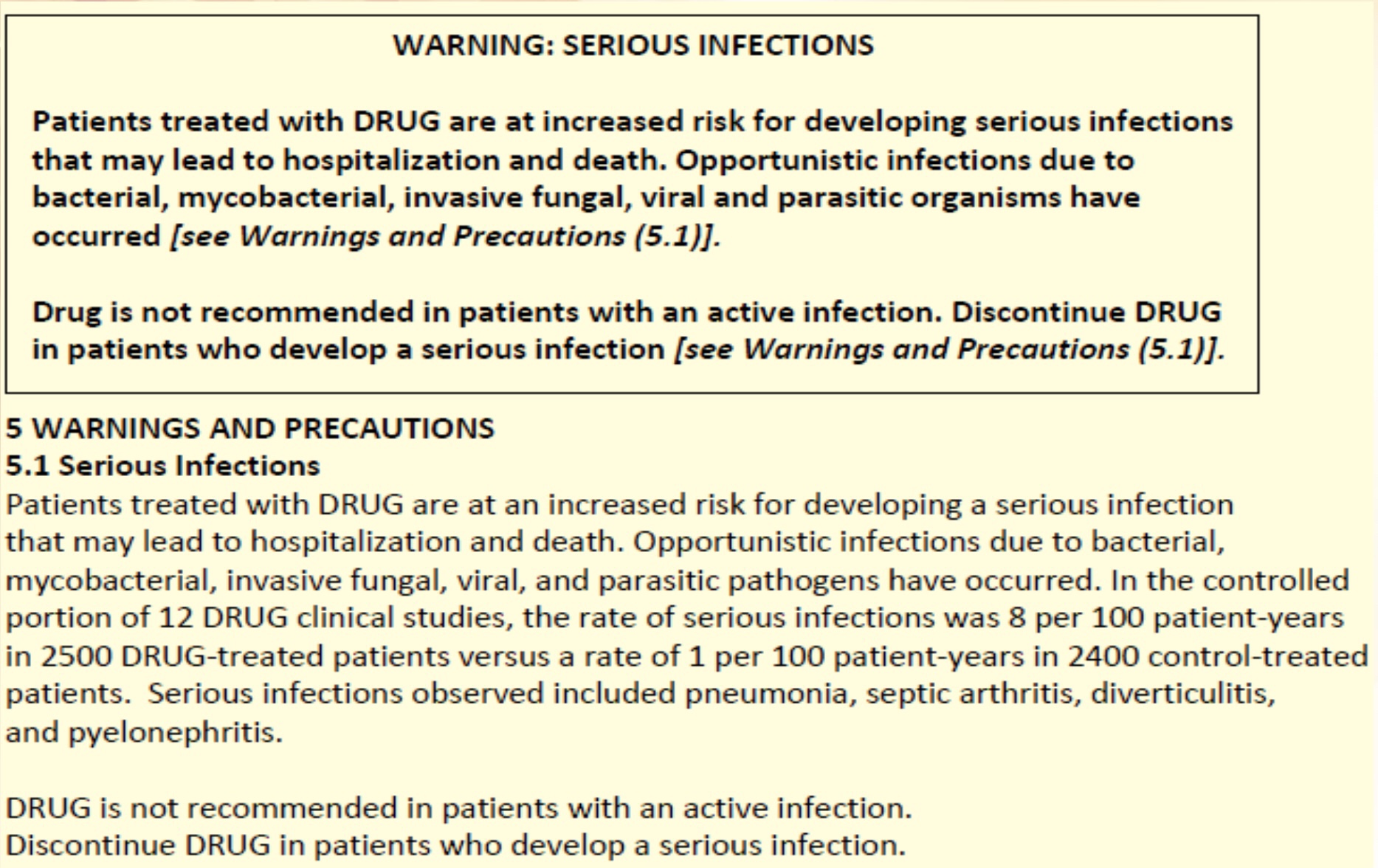
Prescription drugs & boxed warnings
According to the FDAs , a boxed warning is required when a drug carries a risk of serious or lifethreatening adverse events. The text must be placed in a black border and be the first element in the Warnings and Precautions section.
Tobacco products & nicotine warning label
Every pack of cigarettes in the United States must display the rotating set of health warnings on cigarette packets for example, Smoking causes lung cancer. The FDA updated these rules in 2023 to require graphic images that cover at least 30% of the packs visible surface. The same applies to ecigarettes, where the label must also include a nicotine addiction warning.
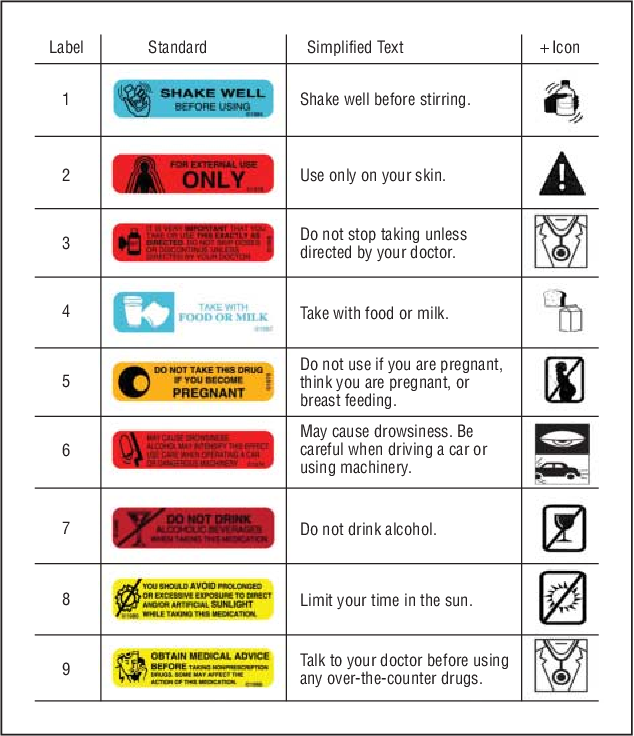
Medical devices, food, and cosmetics
Under 21CFR101.17, any device that presents a potential hazard must include a clear warning statement on the packaging. Food products with known allergens need a bold Contains statement, while cosmetics must list Potentially harmful ingredients if applicable.
Recent updates (2025)
In early 2025 the FDA added a myocarditis warning to certain COVID19 vaccine fact sheets after postmarket surveillance indicated a tiny but significant risk for younger adults. That change illustrates how the FDA warning label ecosystem stays current with emerging science.
Real World Impact
Case Study A: Nicotinereplacement manufacturer
In 2024, a major nicotinepatch producer received a warning letter for not using the updated graphic warning mandated for nicotinecontaining products. After the letter, the company redesigned its packaging, adding a 30% coverage image of a diseased lung. Sales initially dipped, but consumer confidence rebounded because shoppers felt better informed.
Case Study B: Hormone therapy boxed warning debate
When a leading hormonereplacement drug sought to remove its boxed warning, the FDA refused, citing new data on cardiovascular risk. The public discussion highlighted how a label can protect patients even when the manufacturer argues it hurts marketability.
Consumer story: Saved by a warning
I once bought an overthecounter pain reliever that listed Do not take with blood thinners in a small font. My dad, whos on warfarin, read the warning and avoided a dangerous interaction. That tiny line saved him from a potentially lifethreatening bleed.
Staying Compliant
Stepbystep compliance checklist
- Identify product class: drug, tobacco, device, food, or cosmetic.
- Consult the relevant FDA guidance: use the agencys official PDFs or the warningletter database for precedent.
- Draft label text: ensure risk language is clear, concise, and prominent.
- Perform a gap analysis: compare your draft against the FDAs specific label requirements.
- Submit for review (if required): some highrisk products need premarket FDA approval of labeling.
- Implement corrective actions quickly: if you receive a warning letter, address each cited issue within the stipulated timeframe.
Tools & resources
The FDA provides searchable PDFs for most labeling guidance. Bookmark the for drugs, and the for nicotine products.
Common pitfalls & how to dodge them
- Outdated language: FDA updates phrasing regularly always use the most recent version.
- Missing graphic warnings: especially critical for tobacco and nicotine.
- Improper placement: warnings must be on the principal display panel, not buried in fine print.
- Ignoring recent warning letters: the recent FDA warning letters archive is a goldmine for learning what not to do.
Benefits vs Risks
Benefits of clear warning labels
When warnings are accurate and visible, adverse events drop dramatically. Studies show that patients who read boxed warnings are 25% less likely to experience severe side effects. Companies also enjoy reduced liability and higher consumer trust.
Risks of ignoring or misinterpreting warnings
Conversely, a missed or vague warning can lead to recalls, lawsuits, and worst of all, patient harm. The FDAs own data link noncompliance to an average of $23million in direct recall costs per incident.
How you can use warnings to make smarter choices
Before you buy, ask yourself:
- Is the risk statement prominently displayed?
- Does the language use plain English, not legalese?
- Has the product been the subject of a recent FDA warning letter?
Answering yes to the first two questions usually means youre in safe hands.
Final Takeaways
The FDA warning label isnt just a bureaucratic requirement its a safety net woven from years of science, regulation, and realworld experience. By understanding the labels purpose, staying aware of the FDA warning letter ecosystem, and recognizing the balance of benefits versus risks, you protect yourself and help push the market toward greater transparency.
If you found this guide helpful, why not share your own experiences with warning labels in the comments? Have you ever been saved (or nearly harmed) by a label? Lets keep the conversation going together we can make the marketplace a little safer for everyone.
For more on medication risks and common adverse effects, see how anti-androgen side effects can influence labeling decisions for hormone therapies.
FAQs
What is an FDA warning label and why is it required?
An FDA warning label is a mandated statement on a product’s packaging that alerts users to serious risks, contraindications, or required precautions. It is required to protect public health and ensure manufacturers clearly communicate known hazards.
How does a boxed warning differ from a regular caution label?
A boxed warning (often called a “black box”) is reserved for life‑threatening or severe risks and must appear in a prominent black border. A caution label covers lower‑severity issues and is presented in smaller, less conspicuous text.
Where can I find FDA warning letters and why should I read them?
The FDA maintains a searchable warning‑letter database on its website. Reading these letters helps you see real‑world compliance failures, understand current regulatory expectations, and avoid repeating the same mistakes.
What are the main labeling requirements for tobacco and nicotine products?
Tobacco and nicotine products must display graphic health warnings covering at least 30 % of the principal display area, include specific text about addiction, and place the warning on the front and back of the package.
How can businesses ensure their products stay compliant with FDA warning label rules?
Follow a step‑by‑step compliance checklist: identify the product class, consult the latest FDA guidance, draft clear risk language, perform a gap analysis, submit for pre‑market review when required, and act quickly on any warning‑letter findings.