Therapy related AML (tAML) is a type of acute myeloid leukemia that shows up after a person has already been treated with chemotherapy, radiation, or certain immunesuppressing drugs. It accounts for roughly 1020% of all new AML diagnoses and often carries a higher-risk genetic profile. Knowing the warning signs, how doctors diagnose it, and what treatment options exist can help patients and families act quickly and improve outcomes.
Because tAML can develop months to years after an earlier cancer treatment, early recognition isn't just a medical detailit's a lifeline. Below you'll find a friendly, down-to-earth guide that walks you through everything you might be wondering about therapy related AML, from who's most at risk to what the latest therapies can do for you.
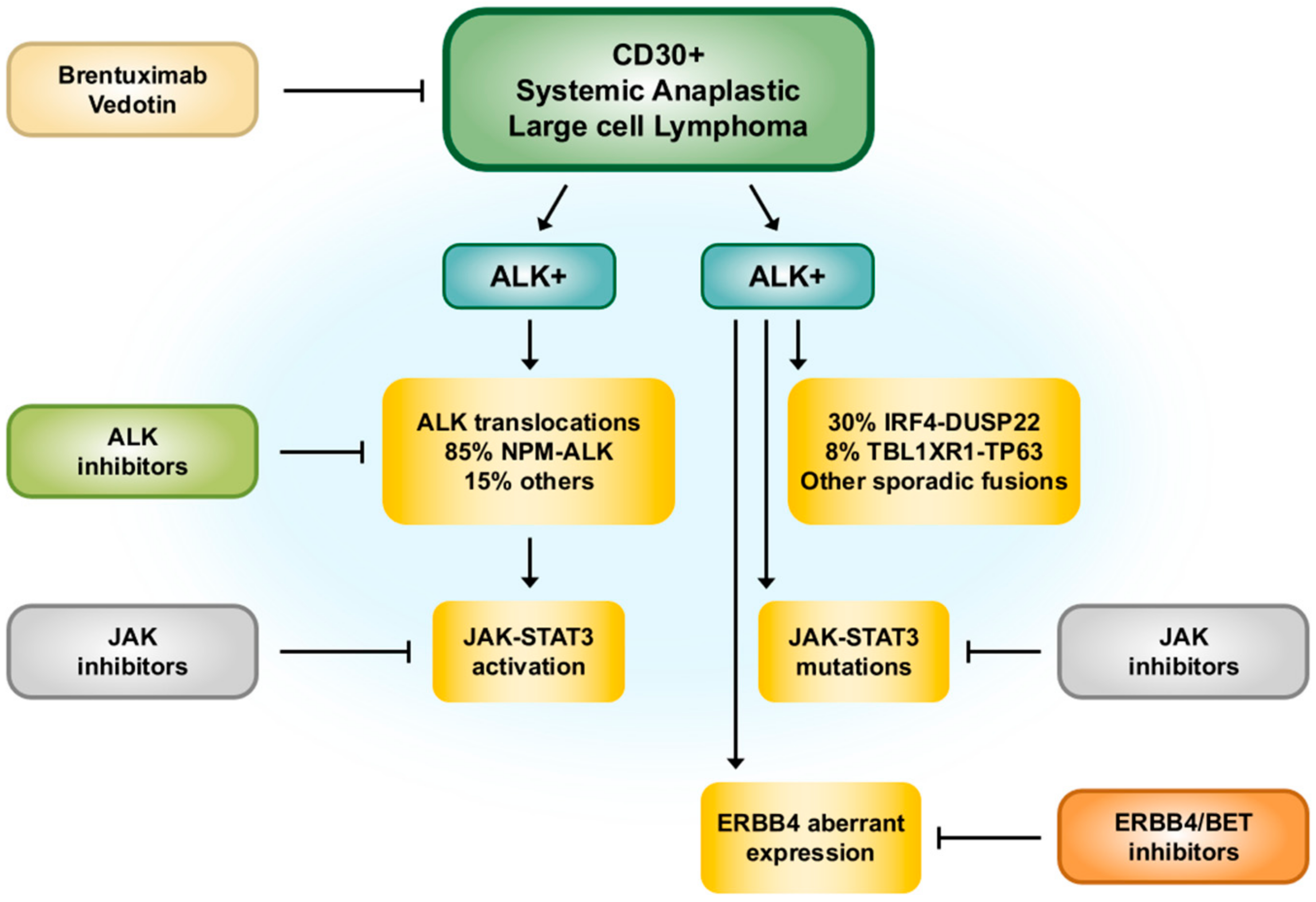
What Is tAML?
Definition and How It Differs From DeNovo AML
In plain language, therapy related AML is a leukemia that pops up after a previous therapy for another diseasemost often a solid tumor or a different blood cancer. Unlike denovo AML, which arises without any known prior treatment, tAML carries a distinct genetic fingerprint that reflects the DNA-damaging effects of the earlier therapy.
Quick Comparison
| Feature | TherapyRelated AML | DeNovo AML |
|---|---|---|
| Typical Origin | After chemo, radiation, or immunosuppressants | No known prior exposure |
| Common Cytogenetics | 5/7, complex karyotype, TP53 mutations | Normal or favorable karyotype more frequent |
| Prognosis | Generally poorer, but improving with targeted drugs | Varies widely; many favorable risk cases |
| Age at Diagnosis | Often older adults; can be children | Broad age range, peak in older adults |
Epidemiology
Recent cohort studies estimate that about 1 in 10 newly diagnosed AML patients actually has therapy related AML. The proportion climbs higher in centers that treat large numbers of solid-tumor patients receiving high-dose alkylating agents or topoisomerase II inhibitors.
Who Is at Risk?
Common Preceding Therapies
The culprits most often implicated are alkylating agents (like cyclophosphamide or melphalan), topoisomerase II inhibitors (such as etoposide and doxorubicin), and high-dose total body radiation. Even some newer targeted therapies have been linked to secondary leukemias, though the risk is lower.
Latency Periods
After alkylating agents, the latency period is usually 57 years, but it can stretch beyond a decade. Topoisomerase II inhibitors tend to produce tAML fasteroften within 13 years. Radiation can act on its own or synergize with drugs, shortening the window.
Special Risk Groups
Children who survive a first cancer (especially acute lymphoblastic leukemia) can develop therapy related AML later on. Their genetic landscape sometimes features KMT2A rearrangements rather than the classic TP53 loss seen in adults. Autoimmune disease patients on longterm immunosuppressive regimens also carry a modest increase in risk.
RealWorld Example
Take Sarah, a 12-year-old who beat acute lymphoblastic leukemia at age 5 with a regimen that included high-dose cytarabine and radiation. Six years later, routine blood work revealed a rising blast count, and a bone marrow biopsy confirmed tAML with a KMT2A-MLLT3 fusion. Her story reminds us that vigilance doesn't stop once the first cancer is in remission.
How Is it Diagnosed?
Clinical Presentation
Symptoms can be surprisingly nonspecificfatigue, easy bruising, frequent infections, or unexplained fevers. If you've had prior chemo or radiation, any of these signs should prompt a quick blood count check.
ICD10 Coding
For accurate documentation and insurance purposes, the correct ICD10 code is C92.0 therapy related acute myeloid leukemia. Using this code helps researchers track outcomes and ensures that patients receive the right follow-up care.
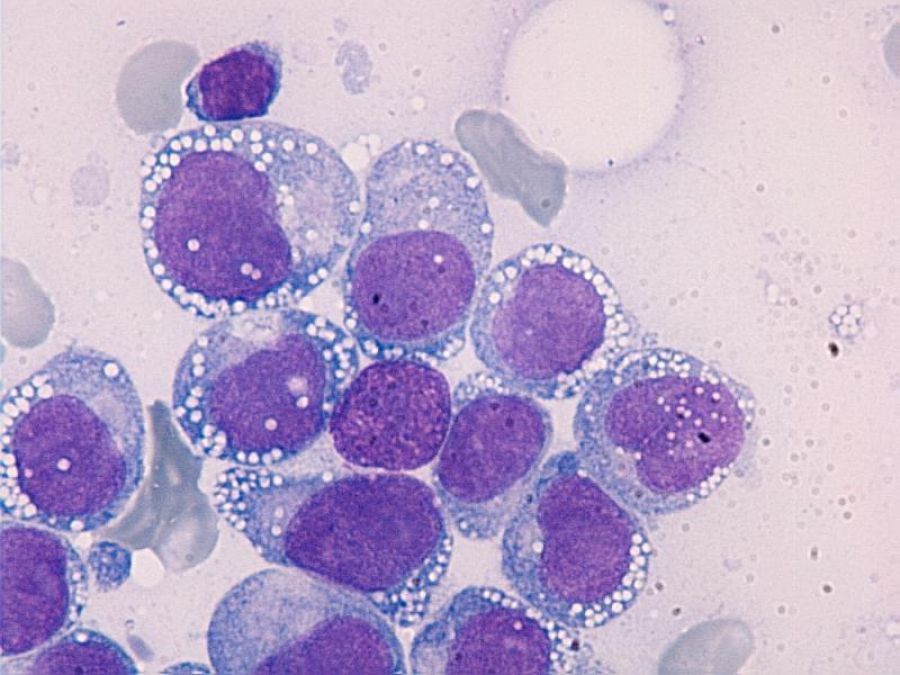
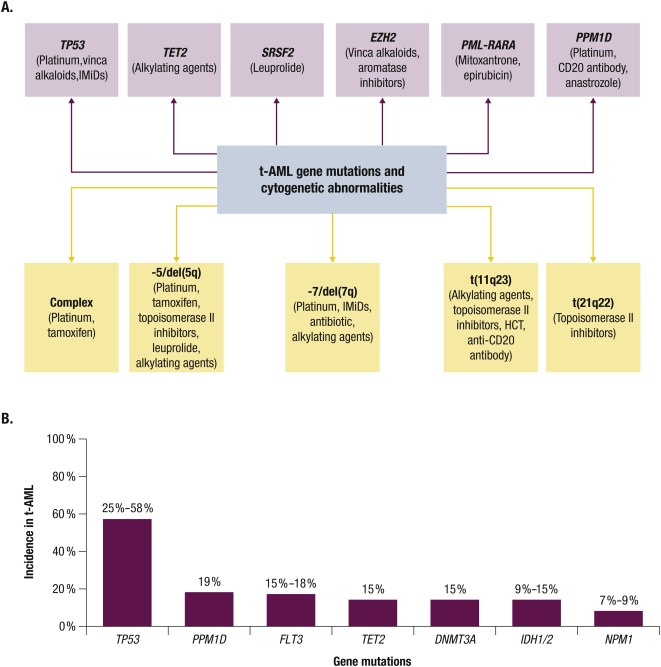
Pathology & Cytogenetics
When the bone marrow sample reaches the lab, pathologists look for the classic myeloblast appearance and run a panel of cytogenetic tests. The most common abnormalities in therapy related AML include:
- Deletion of chromosomes 5 or 7 (5/7)
- Complex karyotype (three or more unrelated chromosomal changes)
- TP53 mutations, often paired with loss of chromosome 17p
- RUNX1, ASXL1, and NPM1 mutations in a smaller subset
These genetic clues are not just academic they directly influence risk stratification and treatment planning.
Reference Study
According to a recent study, patients with TP53-mutated tAML have a median overall survival of just 57 months when treated with conventional 7+3 chemotherapy, underscoring the need for newer strategies.
What Is Prognosis?
Overall Survival & Life Expectancy
Historically, therapy related AML carried a grim outlook, with median survival measured in months. However, the tide is shifting. The introduction of venetoclax-based regimens and FLT3 inhibitors has nudged median overall survival for select patients into the 1224 month range.
Impact of Cytogenetics & Mutations
Risk models such as the ELN 2022 classification weigh your cytogenetic profile heavily. A patient with a complex karyotype and TP53 loss sits in the adverse risk group, while someone with isolated NPM1 mutation (rare in tAML) may fall into a more favorable category.
Prognostic Scoring Tools
Clinicians often combine ELN risk categories with the NCCN guidelines to decide whether a patient is a candidate for intensive chemotherapy, a lower-intensity regimen, or upfront stem cell transplant.
Treatment Options Overview
Intensive Chemotherapy (7+3)
The classic 7+3 regimenseven days of cytarabine plus three days of an anthracyclineremains a backbone for fit patients, but response rates are modest in the adverse-risk tAML population.
Targeted Therapies & New Agents
Enter the era of precision medicine. Venetoclax combined with hypomethylating agents (azacitidine or decitabine) has become a go-to for many older or unfit patients, delivering complete remission rates up to 70% in some trials.
Drug Comparison Table
| Drug Regimen | Indication | Key Trial (PMID) |
|---|---|---|
| Venetoclax+Azacitidine | tAML, unfit for intensive chemo | 2022, PMID35729201 |
| Midostaurin (FLT3 inhibitor) | FLT3-mutated tAML | 2021, PMID33671592 |
| Gilteritinib | Relapsed/refractory FLT3-mutated AML | 2020, PMID32666673 |
Allogeneic StemCell Transplant
For patients classified as high-riskespecially those with adverse cytogeneticsan early referral for allogeneic transplant can be lifesaving. The transplant offers a chance at durable remission, though eligibility hinges on age, organ function, and donor availability.
Clinical Trials
Because therapy related AML remains an area of intense research, enrolling in a clinical trial can provide access to cutting-edge agents like menin inhibitors or novel epigenetic modifiers. Talk to your oncologist about any open studies; many centers now list them on ClinicalTrials.gov.
tAML in Children
Incidence & Unique Genetics
While less common than adult cases, pediatric therapy related AML represents a serious late effect of childhood cancer treatment. Genetic drivers often involve KMT2A (MLL) rearrangements, which differ from the TP53-centric profile of adult tAML.
Pediatric Treatment Protocols
Children are usually treated on collaborative group protocols such as those from the Children's Oncology Group (COG). These regimens balance intensive chemotherapy with the goal of minimizing long-term organ toxicity.
LongTerm Survivorship
Survivors face unique challenges: late cardiac effects from prior anthracyclines, secondary solid tumors, and psychosocial impacts. Dedicated survivorship clinics can help monitor heart health, endocrine function, and mental wellbeing.
Coding & FollowUp
Accurate ICD10 Entry
When documenting tAML, be sure to use the specific code C92.0. This not only ensures proper billing but also contributes to national cancer registries that track secondary leukemia trends.
Recommended FollowUp Schedule
After completing induction therapy, most oncologists schedule:
- Peripheral blood counts every 12 weeks until stable.
- A bone marrow assessment at day 28 to confirm remission.
- Minimal residual disease (MRD) testing every 3 months for the first year.
- Long-term monitoring for late effects (cardiac echo, pulmonary function) annually.
PatientCentered Resources
Finding the right support can make the journey a little less lonely. Organizations like the Leukemia & Lymphoma Society, the American Cancer Society, and disease-specific Facebook groups offer peer-to-peer mentorship, financial counseling, and up-to-date research newsletters. Patients can also explore supportive information on leukemia pregnancy treatment for unique considerations in pregnancy-related cases.
Conclusion
Therapy related AML is a challenging diagnosis, but it's not a dead end. Understanding who's at risk, how the disease is diagnosed, and which modern therapies can improve survival empowers patients and families to make informed decisions. Whether you're navigating a new diagnosis, supporting a loved one, or simply learning for future peace of mind, remember that early detection, precise genetic testing, and personalized treatment plans are your strongest allies.
If you have questions about your own risk, the meaning of a particular genetic result, or how to access a clinical trial, don't hesitate to ask your hematologist. Sharing your story can also help othersfeel free to leave a comment or reach out to a support group. Together, we can turn a scary medical term into something we understand, manage, and, ultimately, overcome.
FAQs
What are the common symptoms of therapy related AML?
Typical signs include fatigue, easy bruising or bleeding, frequent infections, fever, and unexplained weight loss. Any of these after prior cancer treatment should prompt a blood count check.
How is therapy related AML diagnosed?
Diagnosis involves a complete blood count, bone‑marrow biopsy, and cytogenetic testing to identify characteristic abnormalities such as deletions of chromosomes 5 or 7 or TP53 mutations.
What treatment options are available for patients with therapy related AML?
Options range from intensive chemotherapy (7 + 3) to venetoclax + hypomethylating agents, targeted drugs for specific mutations, and allogeneic stem‑cell transplantation for eligible candidates.
Does therapy related AML have a poorer prognosis than de‑novo AML?
Generally yes; adverse cytogenetics and TP53 mutations lead to lower survival rates. However, newer targeted therapies are improving outcomes for many patients.
Can children develop therapy related AML and how is it treated?
Children can develop t‑AML, often with KMT2A rearrangements. They are treated on pediatric protocols such as those from the Children’s Oncology Group, balancing effective therapy with long‑term toxicity concerns.